A QUANTUM SPIN FILTER
In the next page, Quantum entanglement and wave function collapse, I am going to use what I shall call a quantum spin filter to show why the “Copenhagen interpretation” of wave functions apparently collapsing is wrong. (This will lead on to the Many Worlds interpretation which will fall in its turn because of special relativity.) So this page explains the quantum mechanics of spin filters.
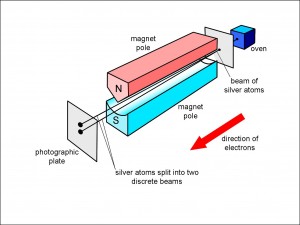
The story starts with Otto Stern and Walther Gerlach in 1922 who had been trying to show that silver atoms had a magnetic field because of electrons that they imagined “orbiting” round the nucleus. They used silver atoms emerging from a hot oven because these atoms make deposits on photographic plates that are clearly visible. What they didn’t know then was that the silver atom has no overall magnetic field attributable to the orbiting electrons because all of the orbital magnetic fields cancel out exactly.
The same is true of the spins of 46 of the 47 electrons in a silver atom. Each electron has a spin, but, in silver, these 46 electrons come in 23 pairs. The spins in each pair, and, therefore, the intrinsic magnetic fields in each pair, cancel out exactly. What is left is the lone 47th electron, which, although it happens not to generate any orbital magnetic field, does have its own intrinsic magnetic field due to its spin.
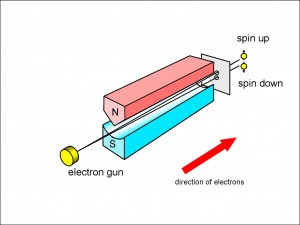
Figure 1 illustrates the experiment carried out by Stern and Gerlach.
The hot silver atoms emerging from the oven passed through a small hole that fashioned them into a beam. This beam was aimed between the poles of an oddly shaped magnet. One of the poles of the magnet had a sharp point so that the magnetic field would be stronger close to that pole than at the other pole. This meant that each atom would be deflected either upwards or downwards, depending upon how the magnetic field of the atom was orientated with respect to the large magnet.
As you can see, two black splodges were found on the photographic plate which meant that the silver atoms were being split into two beams. (In fact, the atoms passed through an elongated horizontal slit rather than a small hole, so that the splodges were smeared out horizontally, but I have removed that incidental complication to make the effect clearer.) Stern and Gerlach assumed this splitting vindicated Niels Bohr’s theory that the orbiting electrons would have discrete values of angular momentum and, hence, magnetic field. What they couldn’t know at the time was that there is, in fact, no overall orbital momentum for silver, and that what they were seeing was the intrinsic spin and magnetic field of the lone 47th electron.
This experiment highlights the weirdness of quantum reality quite well, and so it’s worth analysing just what was going on.
Figure 2 shows the magnet end-on. Look at the magnified electron drawn between the two poles. Using the old, classical physics (i.e., before a better description of quantum properties was available), I have shown the electron spinning in an anti-clockwise direction (i.e., anticlockwise as seen from above). By convention, an electron in the configuration shown in the figure is called a spin-up electron. Since the electron is negative, the equator can be thought of as an electron current going round a loop in an anti-clockwise direction (think of the block arrow as a circular wire carrying this electron current). Such a current generates a magnetic field and the spinning electron behaves like a tiny magnet orientated as shown.
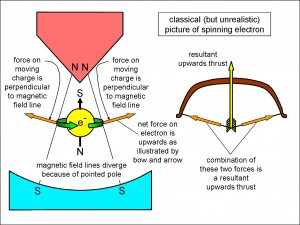
(In fact, we know that the electron cannot physically be spinning like this. Modern experiments show that it is tiny – no bigger than a millionth of a millionth of a millionth of a metre and quite possibly smaller. Therefore, in order to generate an electric current strong enough to create the magnetic field that explains the wavelengths of light emitted by hydrogen, the surface of the electron would have to move round faster than the speed of light! As we have seen, this can’t happen – nothing can travel faster than the speed of light, locally – and so the classical picture of the electron as a spinning object must be wrong.)
Nevertheless, we can still use that picture to give a hand-waving account of what happens in the Stern-Gerlach experiment. Their apparatus was designed to concentrate the magnetic field at one pole and spread it out at the opposite pole as you can see in Figure 2. This means that the magnetic field lines diverge from top to bottom in the figure, and so they are generally not vertical but inclined slightly as shown.
Now, in classical physics we learn that, when a wire is carrying a current in a magnetic field perpendicular to it, the wire feels a force at right angles both to itself and to the direction of the magnetic field. You can see the effect of such a current around the electron as illustrated by the force represented by the two block arrows pointing outwards and upwards from the electron. The outwards push of the arrows in the left and right directions is balanced but there is nothing to balance the upwards push and so, just as two taut strings push upwards on the arrow in the bow on the right of Figure 2, so the electron feels an upward force towards the region where the magnetic field of the magnet poles is most concentrated.
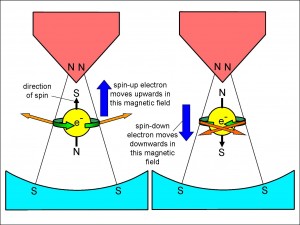
On the other hand, of course, the electron may be spinning in the opposite direction. In Figure 3, you can see what happens in that case. On the right of the figure, this time, the electron is shown spinning anti-clockwise from beneath. We call this a spin-down electron. Since the electron is spinning in the opposite sense, it is easy to see that the forces, represented by the two block arrows, are in the opposite directions to those for a spin-up electron. In particular, the overall effect of the forces is to push the electron downwards, away from where the magnetic field of the poles is most concentrated.
So we appear have an explanation of the two splodges observed by Stern and Gerlach in their experiment. The upper splodge is where atoms containing a spin-up electron were forced, and the lower splodge is due to atoms containing a spin-down electron.
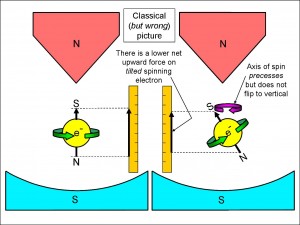
But now we see something extraordinary! What about all the electrons that don’t just happen to be spinning in exactly the upwards direction or in exactly the downwards direction?
In the right half of Figure 4 we have an electron that doesn’t have a vertically inclined spin. This is the most likely case when the silver atoms leave the oven – in general, they will be randomly inclined to the vertical. Now the size of the force on the electron (and, hence, on the silver atom that it is part of) depends on how nearly vertical the spin is.
We have seen that if you turn the spin right over so that a spin-up electron becomes spin-down, the force changes from an upwards one to a downwards one. If, however, the spin is inclined like that of the electron in the right half of the figure, the force will still be upwards, but just not as much as when the electron spin is fully vertical. The size of the upwards force depends on how much of the spin is upwards, which you can see projected onto the ruler. In this picture, the projection of the electron spin on the right of the figure (shown by the vertical arrow on the right-hand ruler) is a little shorter than the projection of the electron spin on the left of the figure (shown by the vertical arrow on the left-hand ruler).
So the projection of the spin is a measure of the force upwards or downwards. You might say – oh well, when the electron spin is not vertical, it experiences a twist from the magnets trying to force it to be vertical, and that will change the direction of spin to a vertical spin-up. However, because the electron is spinning, that twisting force can’t straighten up the electron to spin vertically. A spinning gyroscope will not fall down even although it experiences a twist from gravity – instead, it precesses: the spin axis itself starts to spin. I have indicated this precession with the block circular arrow in the right half of the figure.

So the effect of the magnet on an electron whose spin is inclined to the vertical direction is not to flip it vertically, but to start it precessing. Because of this, the projection of the spin onto the vertical ruler will always be less than if it were purely vertical spin-up. So, if the atom leaves the oven with an electron spin inclined at an angle to the vertical, that angle will not change while the spin precesses, and the force upwards or downwards will be less than for a vertically orientated spin-up or spin-down electron.
Remember that this is what would happen if electrons obeyed classical physics, and, indeed, actually had spins when they left the oven. We shall see in a moment that they do not!
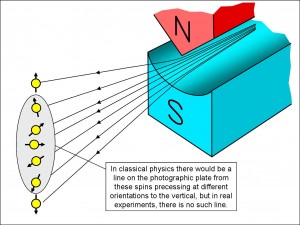
Now look at Figure 5. It shows what happens when the hot silver atoms with their classical electrons leave the oven and travel between the poles of the Stern-Gerlach magnet. Those electrons that happen to be either exactly spin-up or exactly spin-down are pulled to the very top or the very bottom of the array shown to the left of the figure, where they are detected on the photographic plate.
However, if classical physics is correct, an atom that has an electron spin inclined somewhat to the vertical will travel between the magnet poles without the inclination to the vertical changing – the spin will merely precess. So that atom will be forced a little way up or a little way down, but it won’t be forced as far up or down as the atoms with purely vertical spin-up or spin-down electrons.
So, if classical physics is correct, we expect to see a vertical line of deposited silver atoms on the photographic plate. But we don’t – instead there are just two splodges at the points where you would expect to see atoms containing purely vertical spin-up or spin-down electrons.
This is where the quantum world parts company with the old, classical one. If you think of the electrons in the silver atoms having spins before they enter the magnets, then, if they indeed had spins, they would be randomly orientated in all directions, and yet the magnets somehow cause all of the spins either to be pointing fully upwards or fully downwards. Remember: this cannot happen classically – any electron spin that is not fully upwards or downwards would remain that way – it would simply precess and wouldn’t flip into the purely vertical direction.
Now we can use this separation of electrons with opposite spins to make a spin filter.
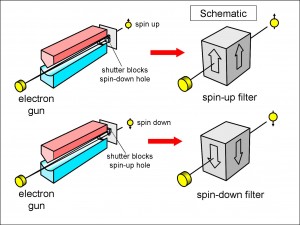
Look at Figure 6. This is just the pair of Stern-Gerlach magnets of Figure 1 again, except that we are looking at it from the other end, and the particles are travelling from left to right in this diagram and not the other way round. Instead of a photographic plate, we have a screen with two holes in it: one for each of the two possible orientations of the electron, spin-up and spin-down. Rather than using silver atoms, we imagine that we have an electron gun firing the electrons between the magnets.
(In the original Stern-Gerlach experiment, the silver atoms were neutral, but electrons carry a fixed negative charge. When a charged particle travels through a magnetic field, it is pushed to the side by the magnetic field like a current-carrying wire in an electric motor, but we want the electron to travel straight down the length of the magnets. To achieve this, we can apply a sideways electric field across the electron path, but this is not shown to keep the diagram simple.)
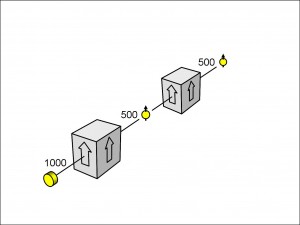
Since the spin-up and spin-down electrons emerge through different holes, we can block either hole to make the apparatus a filter for passing only spin-up electrons or only spin-down electrons (see Figure 7).
In this figure, I have drawn a schematic box alongside each pair of Stern-Gerlach magnets. A box with an arrow pointing upwards represents the apparatus with the spin-down hole blocked, so that it passes only spin-up electrons, and vice versa. You can think of a box, and the apparatus that it represents, as a filter, passing only electrons with a spin pointing in the direction of the arrow. Note that the electrons don’t have to enter the magnets having emerged from the electron gun without a pre-existing spin. They could, instead, be from a source with a pre-existing spin and the filter would work just as well.
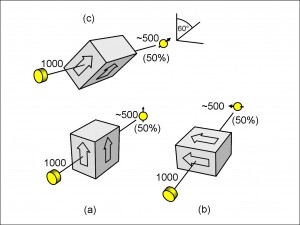
In Figure 8, I have drawn three spin-filters, each with 1000 electrons passing through them from the electron gun. In Figure 8(a), you can see the same spin-up filter as in Figure 7. There are 500 electrons – 50% of the incident number – emerging from the filter as spin-up electrons. Now it should not be surprising that the Stern-Gerlach magnet separates electrons into two equal populations: 50% spin-up and 50% spin-down. Anything else – say 60% and 40% – would beg the question: why? Simply from symmetry, the two populations are going to be equal because there is nothing to make them unequal: there is no connection, for example, between the electron gun and the filter to predispose the incident electrons to spin in any favoured orientation of up or down.
In practice, of course, not every one of those spin-up electrons would emerge from the filter without hitting the sides or otherwise getting lost. In real life, such losses do happen, but ours is a thought experiment, and we can take these losses into account. So, in the laboratory, we should have to do a little more work, like calibrating our filters to make up for the measured proportion of losses.
As a concession to the real world, I have added the tilde sign (“~”) to the numbers emerging from the filters. The sign means that a number is approximate. Sometimes the number will be 527; sometimes it will be 484 and so on. The numbers of electrons emerging will approach 50% of the incident numbers (allowing for the known losses) more and more closely as the incident numbers increase.
In Figure 8(b), I have tipped the filter onto its side horizontally. As before, the electrons emitted from the gun have no favoured spin orientation, and so, again, we should not be surprised to find, after the filter has made a horizontal measurement, that 50% of the electrons emerge with a horizontal spin pointing to the left (as we view it).
Except – maybe we are surprised. Up until now we haven’t gone into the question of why the electrons happened to have a spin that was aligned either parallel or anti-parallel to the direction of the magnetic field of the apparatus. Now Figure 8(b) forces the question – is the apparatus itself aligning the spins of the electrons? It certainly looks that way.
This is where the weirdness of quantum mechanics creeps in again. Strictly speaking, when the electrons enter the filter from the electron gun, they don’t even have a spin in any particular direction, and so there is no spin for the apparatus to work on! You shouldn’t imagine that the electrons are leaving the electron gun each with a particular spin orientated randomly in any direction so that, on average, after many electrons have passed into the apparatus, there is no overall direction of spin. No! – when each electron leaves the electron gun, it doesn’t have a spin in any direction at all!
Now, you may be thinking: OK, we admittedly don’t know anything about the direction of the spin of any individual electron when it leaves the gun, but it must surely have a spin, even if we haven’t measured it yet! Well, you are in good company, because this is exactly the way that Einstein thought about the problem of quantum mechanics: if electrons are real, then they must possess the elements that make them real, such as mass, charge and spin, even if we don’t measure them all the time. Since quantum mechanics cannot tell you about these properties unless we measure them, he argued, then quantum mechanics is incomplete. It cannot tell you about the hidden variables (as they were later to become known).
At the risk of spoiling a good story, but in order not to let you build up misconceptions if you don’t know the answer already, it will turn out that reality has no hidden variables (at least, as I shall explain later, none that act only locally). In the meantime, to answer the question about what the apparatus is doing when we change its orientation, it is simply defining a direction, nothing more. So, when the electrons pass through the apparatus after leaving the electron gun, this is the first measurement made on them, and, for the first time in the experiment, they have a spin, and that spin is split 50:50 along the direction defined by the filter, whether it is inclined vertically, horizontally or at some other angle.
Just to emphasise the point, in Figure 8(c) I have drawn a filter inclined at an angle of from the vertical, and, again, we find that, in defining such an axis, 50% of the electrons are orientated in the spin-up direction along this axis, and 50% are in the spin-down direction.
We can test the idea that the electrons now have a spin, having passed through a filter: we can place a second filter after the first, and see how many come through. If we measure the electrons’ spins in the vertical spin-up direction using the appropriate filter, we can place an identical filter downstream and count the number of electrons emerging from this second filter.
The experiment is shown in Figure 9.
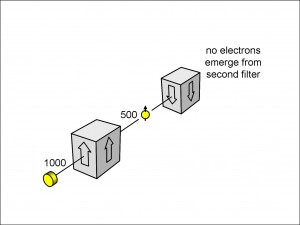
Here, 500 electrons with a vertical spin-up are shown emerging from the first filter and entering the second, identical filter. (Note that I have left out the “~” sign this time: you can think of the number 500 as what you would find after averaging the result of very many such experiments where you start with 1000 electrons each time.) What we observe is that all of these electrons (once due allowance is made for instrumental losses, as we discussed above) pass through the second filter. In other words, once the electrons have a spin orientation defined by the first filter, they retain that spin orientation, which allows them to pass unimpeded through the identical second filter.
We can check this interpretation by substituting a vertically inclined spin-down filter for the second vertically inclined spin-up filter. As we see from Figure 10, no electrons emerge from the second filter this time, which is what we should expect if the electrons entering it are all vertically spin-up. Such a filter would trap all of these electrons and pass none of them.
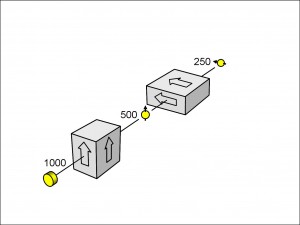
Now that we know that electrons leaving a measuring filter have a spin aligned with the direction of the filter, we can ask the question: what would happen if electrons that are orientated vertically in the spin-up direction enter a filter orientated exactly horizontally? We might guess that, like polarizing plates at right angles to each other that let no light through, no electrons might pass through the crossed filters.
(In fact, it is easier in practice to do experiments with polarized photons than with electron spin. It turns out that the predictions using polarizers work for spin-filters if you double the angle in translating from the angle between polarizers to the angle between spin-filters.)
On the contrary, though, Figure 11 shows what happens.
As you see, when the electrons leave the first filter with a vertically orientated spin-up and enter the second, horizontally inclined filter, half of them pass through it, orienated in the horizontal spin-up direction. (You could equally call this the horizontal spin-down direction as long as you called the opposite direction spin-up.)
If you repeat the experiment in Figure 11 with the horizontal spin-filter orientated in the other direction, you will again get half of the incident electrons passing through it, this time aligned in the other direction.
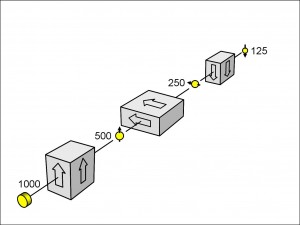
Now here is a fundamental question: In Figure 11, since the electrons entering the horizontal filter are already orientated in the vertical spin-up direction, do they retain their vertical component of spin as well as acquiring the new horizontal component that we detect when they emerge from the horizontal filter? Let us see. We can add a third filter after the two in Figure 11, this time aligned to pass only vertically orientated spin-down electrons. If each of the electrons emerging from the horizontal filter with horizontal spin has also retained the vertical spin-up component, then none of them should get through the final filter. Figure 12 shows the result of the experiment.
As you can see, fully half of the horizontal-spin electrons pass through the final filter, emerging with a vertical spin-down orientation. This is not what you expect if the electrons entering the final filter had a vertical spin-up component, but it is exactly what you would expect if they had entered the final filter with only a horizontally aligned spin. In fact, you can check that by repeating the experiment with the middle filter horizontal again, but pointing in the opposite direction. In this repeat experiment, you find that, again, one half of the electrons pass through the final filter.
The implication of such experiments is that electrons cannot possess any component of spin in any direction other than the one being measured: the very act of measurement destroys any other component. (In fact, this is a manifestation of the famous Heisenberg uncertainty principle.)
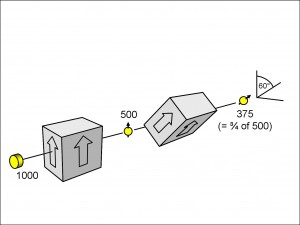
There is another question we can ask: is there a general rule for the number of electrons that pass through a filter that is inclined at any angle, say theta ? Yes, there is, and, since we shall want to use the answer to this shortly, let us take a specific example where the angle theta is 60 degrees (see Figure 13).
In this experiment, we have prepared 500 electrons in the vertically orientated spin-up direction and passed them through a second filter inclined at 60° to the vertical. We find that, after averaging over many experiments, three-quarters of the electrons incident upon the second filter pass through it, aligned now, of course, at 60 degrees to the vertical in accordance with the inclination of the filter. Indeed, regardless of how the first filter is aligned in space (vertical, horizontal or whatever), as long as the second filter is inclined at 60 degrees to it so that the spin-up directions are at 60 degrees to each other, then three-quarters of the electrons pass through it.
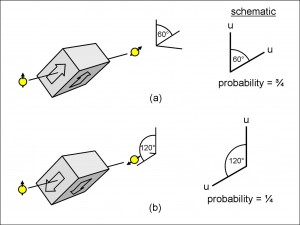
We are going to be using these particular geometries again in the next page, and so it is worth making shorthand pictures of them to make things clearer. In Figure 14(a), I have reproduced the second filter of Figure 13 and added a schematic drawing showing the geometric relation between the spins of the electron when it is incident upon the spin-filter and when it leaves it, together with the probability that this will occur.
The schematic drawing shows two lines at an angle of 60 degrees, the vertical one representing the orientation of the electron spin as it enters the inclined spin-filter, and the inclined line representing the orientation of the electron spin when it has left the spin-filter. I have used a convention in the schematic drawing to indicate whether the electron is spin-up or spin-down. If the spin of the electron points away from the vertex between the two lines, then it is spin-up and this is indicated by a “u” at the end of the line. If the spin of the electron pointed towards the vertex, then it would be spin-down, indicated by a “d”.
In Figure 14(a), the incident electron enters the spin-filter with a vertical, spin-up orientation, and this is shown with a “u” at the end of the vertical line in the schematic drawing. The electron shown emerging from the spin-filter is inclined at 60 degrees to the vertical, but still with a spin-up orientation, and this is shown again with a “u” at the end of the 60 degree line in the schematic drawing. The schematic has been labelled with the probability for the electron to emerge with this orientation, which, as we saw above, is three-quarters
Since all the electrons will have been inclined at 60 degrees by travelling between the Stern-Gerlach magnets of the spin-filter (that is, they will all be either spin-up or spin-down, but inclined at 60 degrees), the probability that they are in the spin-down orientation is one quarter, as we saw above. We can make this more explicit by inverting the orientation of the spin-filter in Figure 14(a) so that it appears as in Figure 14(b). We can either think of this as the spin-filter with the same 60 degree inclination but with a spin-down orientation, or, equally, we can think of it as a spin-filter aligned at 120 degrees with a spin-up orientation. I have chosen the latter view for the schematic in Figure 14(b), and I have shown the probability as one quarter.
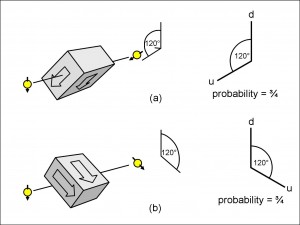
In Figures 15(a) and (b), you can see experiments with the same geometry except that in both cases the vertically inclined incident electron is now orientated spin-down.
Compare Figure 14(a) with Figure 15(a) and you will see that the latter experiment is made simply by rotating the whole of the former experiment through 180 degrees around the axis of travel. Therefore, the probability of the electron emerging is still three-quarters. I have also re-labelled the vertical axis: instead of rotating it, as I did with the inclined axis, I have kept the vertically up line but called it “d” instead of “u”.
Figure 15(b) is just the mirror image of Figure 15(a), with the same probability of three-quarters.
This completes the description of the quantum spin filter that I am going to use in the next page to show you quantum entanglement.
Click here to go to [6] Quantum Entanglement
